FIRST COMBINATION OF A NON-ELECTRIC INFUSION PUMP WITH SELECTABLE RATE FLOW CONTROL FOR INTRAVENOUS AND SUBCUTANEOUS USE.
INTRAVENOUS INFUSION THERAPY
Safe, effective, simplified, groundbreaking non-electric medication delivery.
SUBCUTANEOUS INFUSION THERAPY
A new medical paradigm: convenience, safety, innovation, and autonomy as the patient now can select, titrate, and/or modify the flow rate in real-time
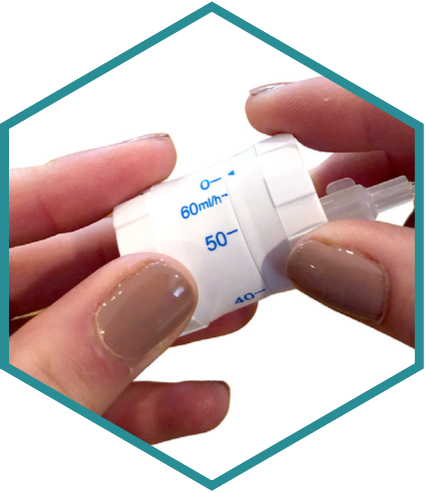
New Infusion Approach: Better Infusion Performance.
The Insignis™ system delivers the performance, convenience, and accuracy of electronic systems and is designed to be:
- Portable
- Cost-effective
- Mechanical
- Simple to use
- Highly accurate
- Safe, low pressure
- Versatile
- Reliable
Learn more about our products
A Company Purposefully Built to Advance Infusion Technologies
In 2019, renowned medical engineer and inventor Andy Sealfon launched IHS to make a meaningful difference for patients worldwide. Some of these patients-who require ongoing lg replacement, or IV antibiotic therapy-were burdened by equipment that was expensive, hard-to-use and often imprecise, which hindered their mobility or made it difficult to administer and control their treatments.
Today, Innovative Health Sciences [IHS] is solving these problems at the forefront of infusion technology. We are creating cutting-edge, innovative products – like the Insignis Syringe Infusion System – that bring measurable value throughout the entire infusion market.

News
IHS Passes Rigorous Audit for World-Wide Distribution of its Insignis™ Infusion System
Innovative Health Sciences has successfully secured the exclusive Medical Device Regulations Quality Management System Certificate from BSI (CE MDR QMS), a leading notified body, thus facilitating the introduction of the Insignis™ Infusion System into the world...
IHS Expands Team to Market Insignis™ Syringe Infusion System after Receiving FDA Clearance
CHESTER, N.Y., Aug. 5, 2024 /PRNewswire/ -- Innovative Health Sciences ("IHS" or the "Company"), a medical device company that creates state-of-the-art drug infusion solutions, announced today the expansion of its commercialization team to market the Insignis™ Syringe...
Innovative Health Sciences Receives FDA Clearance for Insignis™ Subcutaneous Needle Sets
CHESTER, N.Y. (August 26, 2021) – Innovative Health Sciences, LLC (“IHS” or the “Company”) management announced today that IHS has received FDA clearance for its Insignis™ Subcutaneous Needle Sets. This regulatory clearance allows IHS to sell and distribute its...



